The Mean Kinetic Temperature (MKT) is a parameter that allows you to evaluate the effects of temperature changes on the vaccine or drug quality. In case of temporary exceeding of storage temperature limits, it can be determinant of the usefulness of the drug. Mean Kinetic Temperature is important both in the preparation of facilities for storage and distribution, as well as during their operation. MKT is calculated using the following formula.
where:
is the activation energy (in kJ mol−1)
is the gas constant (in J mol−1 K−1)
to are the temperatures in Kelvins
to are time intervals at each of the sample points
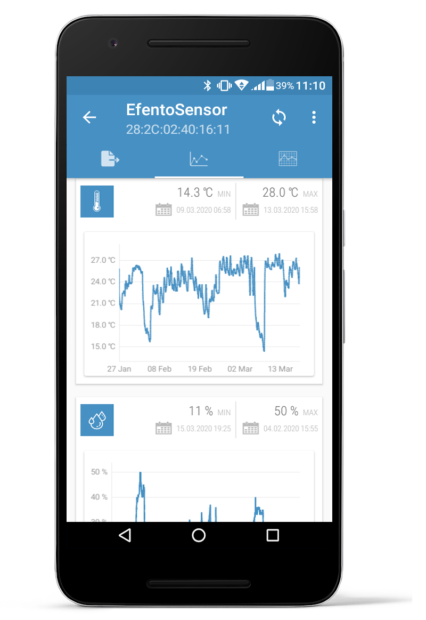
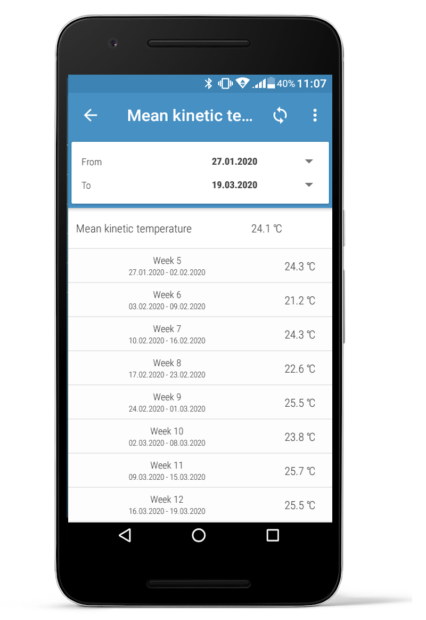
Efento mobile application calculates the Mean kinetic temperature based on the data recorded by Efento sensors. MKT is calculated in the application, so the user does not need to fill spreadsheets or use any dedicated software, as in the case of other systems available on the market.
MKT is calculated from both all measurements taken by the sensor, as well as for each week. In addition, the application calculates the minimum, maximum and average value of the series of measurements.
Moreover, the same information is provided in Efento Cloud PDF / CSV reports for all temperature sensors (both with probes and built in sensors). When exporting the data, select the time period that will be included in the report and Efento Cloud will automatically calculate the MKT value for it.
The object preparation phase
According to the guidelines from Good Distribution Practice [1], distribution and storage of medicinal products can only take place with the use of vehicles and warehouses, in which an analysis of the temperature distribution (mapping) was made.
Before using the storage area, an initial temperature mapping in representative conditions should be carried out. Temperature monitoring equipment should be distributed according to the results of the mapping, so that the monitoring devices are in areas where temperature fluctuations are the highest.
The purpose of mapping the distribution of temperature and humidity is to define the location of the measurement points (critical positions), and the minimum number of sensors necessary to continue monitoring.
Mapping of the temperature distribution is carried out in several stages:
- Determination of measurement points in the storage area. Sampling points should be chosen to allow the capture of temperature fluctuation, that is, at different levels and in critical areas: near the ceilings, walls, heat sources, doors, loading chambers, etc..
- Collection of measurements. It is recommended that the measurements were carried out for at least one week (including weekends) to capture all the long-term trends in temperature changes depending on the time of the day, work of the staff and equipment, etc..
- Data analysis. Using an Efento Logger, after downloading data from wireless loggers application automatically calculates parameters such as the average kinetic temperature, average temperature, minimum and maximum temperature in the measurement series. Data can be exported in CSV format for further editing and analysis.
In addition, the mapping should be carried out after any change of equipment and installation modifications (air conditioning, ventilation) in the storage/load of the vehicle. Additionally, the mapping should be carried out taking into account the extremes of temperature in both winter and summer.
Operation phase
During operation of facilities average temperature kinetic can be helpful in evaluating whether the drug is consumable in case of exceeding the permissible storage temperature.
According to reference[4], drugs storage conditions are defined as follows:
- Keep in the freezer (-25 to – 10 ° C)
- Store in a refrigerator (2 ° C to 8 ° C), the temperature must not exceed 8 ° C
- Keep “cold” (no more than 8 ° C)
- Keep in a cool (8 ° C to 15˚C)
- Keep at room temperature
- Keep at controlled “cold” (2 ° C to 8 ° C). The average kinetic temperature did not exceed 8 ° C, which means temperature fluctuations in the range not wider than 0 ° C to 15 ° C. If the calculated MKT value is within permitted range and temporarily increases to 25 ° C, it is acceptable, provided that the period of the fluctuation does not exceed 24 hours [4]
- Store at controlled room temperature (20 ° C-25 ° C). The average kinetic temperature did not exceed 25 ° C, which means temperature fluctuations in the range not larger than 15-30 ° C. If the calculated MKT value is within permitted range and temporarily increases to 40 ° C, it is acceptable, provided that the period of the fluctuation does not exceed 24 hours. [4]
- Keep warm (30 ° C to 40 ° C)
- Keep at high temperatures (above 40 ° C)
- Store in a dry place (below 40% moisture at a temperature of 20 ° C)
- Protect from freezing
- Protect from light
References
[1].http://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/com_2013_c68/com_2013_c68_pl.pdf
[2].http://www.ptfarm.pl/pub/File/Farmacja%20Polska/2009/10-2009/05%20%20Mapowanie%20temperatury.pdf
[3].http://www.sensitech.com/assets/articles/mktapopapergalley13april2009.pdf
[4] www.usp.org/sites/default/files/usp_pdf/EN/USPNF/generalNoticesandRequirementsFinal.pdf