Recently, three pharmaceutical companies (Pfizer & BioNTech, Moderna, Astra Zeneca) have announced that they have developed and successfully tested COVID-19 vaccines candidates, which over 90% efficiency. Are there any changes in the storage and transportation conditions of the COVID-19 vaccines (Pfizer) compared to the vaccines used today? Yes. The key change is the temperature, in which the vaccine should be stored. Unlike the traditional vaccines we use today, the new, mRNA based COVID 19 vaccines should be kept in very low temperatures, ranging from -90 to -60°C. Once thawed, the vaccines can be stored in 2 – 8 °C for up to five days (120 hours).
Challenges
In 2021, experts expect that pharmaceutical companies will produce around 10 billion COVID-19 vaccines, and the cold chain must be able to handle this huge increase on top of the vaccines that must be distributed every year already.
Moreover, the logistics companies, hospitals, and health care facilities where the vaccinations will take place will have to remodel the cold chain to transport and store the COVID-19 vaccines in the deep freeze: -80 to -30°C, depending on the vaccine type.
“We’re talking about a vaccine that needs storage at minus 70 or 80. That’s a tremendous logistical issue not only in the US but outside the Western world. We’re a major medical center and we don’t have storage capacity like this. That will be true for everybody. This is a logistical obstacle.”
Dr Gregory Poland, a virologist and vaccine researcher with the Mayo Clinic.
Currently many companies are getting ready for the upcoming challenge. For instance UPS is building giant freezer farms to be able to quickly deliver the vaccine across the globe. The facilities, under construction in the US and the Netherlands, will house a total of 600 deep-freezers that can each hold 48,000 vials of vaccine.
How the typical cold chain for COVID-19 mRNA vaccines is organised?
- Vaccines arrive from the pharmaceutical company to a country in cool boxes filed up with dry ice. Dry ice provides temperature around -80 °C

Vaccines are transported in cool boxes filled up with dry ice (source: Twitter, Kancelaria Premiera RP)
- In each country, vaccines are kept in distribution centres and stores in low temperature freezers in the range of -90 to -60° C.
- Pfizer vaccines are stored in special freezers at temperatures ranging from -90 to -60 C
- The temperature probe is inserted into the freezer through a special validation hole. This ensures maximum tightness and does not affect the operation of the device
- Vaccines are distributed among hospitals and clinics, which vaccinate the people. During the transportation, the vaccines are kept in cool boxes filled with dry ice (-80 ° C)
- Once arrive in the hospital / clinic, a vaccine can be stored in regular refrigerator (2 to 8° C) for up to five days (120 hours)
How to monitor the temperature of the COVID-19 vaccines?
Efento provides low temperature wireless sensors, which can monitor the temperature up to -200 °C. The loggers are based on Pt1000 sensor, which provides excellent accuracy in the deep freeze temperature. Typical accuracy of the sensor in -80 °C is +/- 0.2 °C. Sensor probes are covered with a teflon layer, which products them against long term influence of the extreme temperature. Efento wireless low temperature sensor works with all Efento software applications including Efento Cloud and Efento mobile applications, which allows customers to use it to monitor both transport and storage conditions.
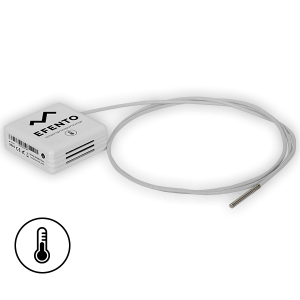
Efento wireless low temperature sensor
Once a vaccine arrives in a hospital / clinic it can be stored in 2-8 °C for up to five days. In that case a regular temperature sensor can be used to monitor its temperature. Efento temperature sensors work with all Efento software applications including Efento Cloud and Efento mobile applications, which allows customers to use it to monitor both transport and storage conditions.

Efento Cloud
Data from the wireless sensor can be stored in Efento Cloud platform. It is an IoT platform that allows you to collect, analyse and visualise sensor data, generate reports out of it and notify users, if the values measured by the sensors are out of the safe range. Efento Cloud works with all Efento sensors, no matter what communication technology they use. Platform offers a RESTful API, which can be used to integrate it with any third party software. Users can access Efento Cloud through a web browser or a mobile application.
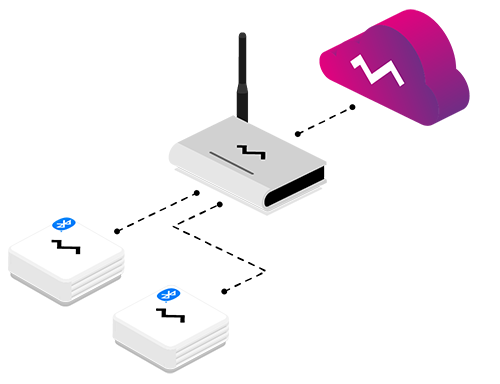
Efento sensors send the data to the Efento Cloud platform, where it’s stored and analysed
Calibration certificates
Efento collaborates with a number of ILAC accredited calibration laboratories. This allows us to provide customers with calibration certificates, which confirm the sensors’ accuracy in the low temperatures. This is required by the international / national regulations in order to confirm the cold chain monitoring system proper operations. You can learn more about the calibration certification in our knowledge base.


